Introduction
Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer cases, making it a significant global health concern. Traditional treatments include surgery, chemotherapy, and radiation therapy; however, these approaches often have limited efficacy, especially in advanced stages. In response to this challenge, researchers have developed Racotumomab, a therapeutic vaccine designed to stimulate the immune system to target and destroy cancer cells.
Understanding Racotumomab
Mechanism of Action
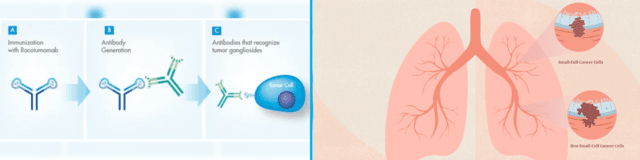
Racotumomab is an anti-idiotype monoclonal antibody vaccine that targets the NeuGcGM3 ganglioside, a tumor-associated antigen expressed on the surface of various cancer cells, including those in NSCLC. By mimicking this antigen, Racotumomab prompts the immune system to recognize and attack cells bearing NeuGcGM3, thereby inhibiting tumor growth.
Development and Approval
Developed through a collaboration between researchers in Cuba and Argentina, Racotumomab has undergone extensive clinical evaluation. It has been approved for use in Argentina and Cuba for the treatment of recurrent or advanced NSCLC, particularly in patients who have exhausted standard therapeutic options.
Clinical Trials and Efficacy
Phase III Clinical Trial
A pivotal randomized, multicenter, placebo-controlled Phase III clinical trial assessed the efficacy of Racotumomab as a switch maintenance therapy in patients with advanced NSCLC. Participants who achieved disease control after first-line chemotherapy were administered Racotumomab. The study demonstrated a significant improvement in overall survival and progression-free survival compared to the placebo group.
Real-World Applications
Beyond controlled clinical settings, Racotumomab has shown promise in routine clinical practice. A retrospective study involving 71 patients with inoperable NSCLC revealed that those receiving Racotumomab as switch maintenance therapy experienced a median overall survival of 17.2 months. Additionally, the vaccine was well-tolerated, with 84.5% of patients reporting no adverse events.
Administration and Safety Profile
Dosage and Administration
Racotumomab is administered via intradermal injection. The typical regimen involves an initial induction phase, with doses given bi-weekly, followed by a maintenance phase with monthly administrations. This schedule is designed to sustain the immune system’s response against cancer cells.
Safety and Tolerability
Clinical studies have consistently shown that Racotumomab is well-tolerated. The majority of adverse events are mild and include localized reactions at the injection site, such as redness and swelling. Systemic side effects are rare and generally transient.
Future Perspectives
Ongoing Research
Research continues to explore the full potential of Racotumomab in NSCLC treatment. Ongoing studies aim to evaluate its efficacy in combination with other therapeutic modalities, such as immune checkpoint inhibitors and targeted therapies. Additionally, investigations are underway to determine its applicability in other malignancies expressing the NeuGcGM3 antigen.
Global Accessibility
While Racotumomab has been approved in Argentina and Cuba, efforts are being made to expand its availability to patients worldwide. International collaborations and further clinical trials are essential to achieve broader regulatory approvals and integrate Racotumomab into standard NSCLC treatment protocols globally.
Conclusion
Racotumomab represents a significant advancement in the immunotherapeutic approach to treating non-small cell lung cancer. By harnessing the body’s immune system to specifically target cancer cells, it offers a promising option for patients, particularly those with advanced disease stages. Continued research and international cooperation are crucial to fully realize its potential and make this innovative treatment accessible to a broader patient population.
References
- A Randomized, Multicenter, Placebo-Controlled Clinical Trial of Racotumomab-Alum Vaccine as Switch Maintenance Therapy in Advanced Non–Small Cell Lung Cancer Patients
- Racotumomab in Non-Small Cell Lung Cancer as Maintenance and Second-Line Therapy
- Racotumomab – Wikipedia
- Racotumomab Completed Phase 2 Trials for Advanced Non-Small Cell Lung Cancer (NSCLC) Treatment